This User Agreement (hereinafter referred to as the “Agreement”) is concluded between Pharmacy Mall (hereinafter referred to as the “Agent”), on the one hand, and the User on the other. The Agent and User are collectively referred to as the Parties. Hereby, Pharmacy Mall declares that it will consider itself to have concluded the Agreement…
Search Results for:
Terms of Use
Privacy Policy
The use by the Client of nephrogenex.com signifies acceptance of this Privacy Policy and the terms of processing the Client’s personal data. By providing his personal data when registering on the website of Pharmacy Mall, as well as using this website, the Client agrees to his data processing by Pharmacy Mall and its partners, agrees…
PYR-311: Pyridorin in Patients with Diabetic Nephropathy
Pivotal program uses new FDA-approvable endpoint The Phase 3 PIONEER program includes two identical double-blind, placebo-controlled Phase 3 trials. Each is designed to evaluate the safety and efficacy of PYRIDORIN® (pyridoxamine dihydrochloride) at 300 mg twice a day compared to placebo in reducing the rate of renal disease progression in Type 2 diabetic patients. PYR-311 is…
Early Studies Show Clinical Benefit for Target Patient Population
Pyridorin well tolerated, with adverse event rates similar to placebo Following several preclinical and Phase 1 studies, four placebo-controlled PYRIDORIN® (pyridoxamine dihydrochloride) Phase 2 trials provided the guidance to support the design of the Phase 3 PIONEER program. This included PYR-206, PYR-205/207, and PYR-210. All four studies suggested a clinical benefit in diabetic nephropathy for patients…
Pyridorin: A Novel Acting Compound to Improve Treatment of Diabetic Nephropathy
Addressing an unmet medical need to slow disease progression At NephroGenex, we are committed to developing a more effective treatment for diabetic kidney disease—a critical global health issue. We are now in a Phase 3 study with PYRIDORIN® (pyridoxamine dihydrochloride), a novel acting compound to reduce the rate of disease progression in patients with diabetic…
NephroGenex, Inc. Commences Voluntary Chapter 11 Proceeding; Seeks To Initiate Sale Process Under Section 363
RALEIGH, N.C.–(BUSINESS WIRE)–NephroGenex, Inc. (Nasdaq:NRX), a pharmaceutical company focused on the development of therapeutics to treat kidney disease, today announced that it filed a voluntary petition under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the District of Delaware (the “Court”). In connection with its decision to seek…
Many Critical Care Patients Are at Risk for Acute Kidney Injury
The condition can result in loss of kidney function in just 1-2 days Acute kidney injury (AKI) is characterized by an abrupt loss of kidney function that usually develops within just a few hours to a few days. AKI occurs when the kidneys suddenly become unable to filter water and waste products from the body…
Chronic Kidney Disease Affects Over One-Third of Diabetics in the U.S.
Patients with long-standing diabetes are at high risk for developing renal failure The kidneys are two fist-sized organs located in the back of the abdomen on either side of the spine, directly above the waist. Our kidneys perform several crucial and life-sustaining functions including removing waste and excess fluid and regulating blood pressure. The two…
Pyridorin: Treating An Underlying Cause of Diabetic Nephropathy
Studies found slowing of disease progression in target population PYRIDORIN® (pyridoxamine dihydrochloride) is a novel small molecule drug candidate with a chemical structure similar to, but distinct from, vitamin B6 (pyridoxine). Unlike vitamin B6, pyridoxamine is regulated as an investigational drug candidate by the U.S. Food and Drug Administration. The compound has a distinct structure…
Mechanism of Action
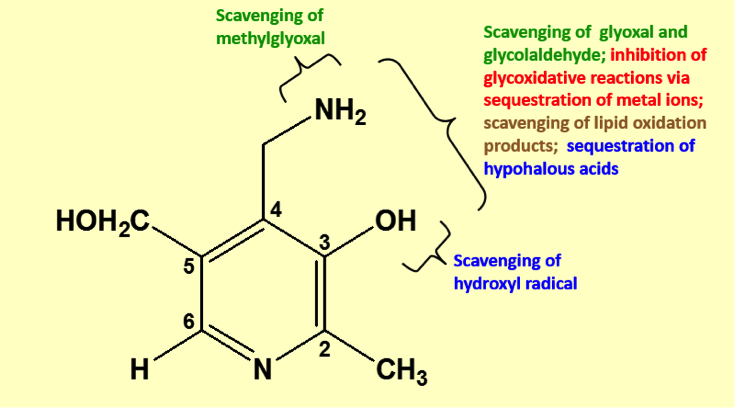
Pyridorin: Unique Mode of Action Inhibits Key Cause of Disease PYRIDORIN® (pyridoxamine dihydrochloride) is a novel small molecule drug candidate with a chemical structure similar to, but distinct from, vitamin B6 (pyridoxine). Unlike vitamin B6, pyridoxamine is regulated as an investigational drug candidate by the U.S. Food and Drug Administration. The compound has a distinct…